Canada’s current approach to the opioid crisis has left health advocates desperate for alternatives.
In response to the growing strain on the healthcare system, Dr. Ryan Patchett-Marble and his team at Marathon Family Health Team in Northern Ontario developed the HARMS Program and START-IT tool. The program features Ocean Tablets and employs a systematic approach to identify at-risk patients and monitor their opioid prescriptions for chronic non-cancer pain through routine urine drug testing (UDT).
UDT remains one of the only objective measures for determining opioid risk. It can objectively identify substance use, which when part of a substance use disorder raises the risk of opioid addiction, overdose and death. A concerning UDT will therefore increase someone’s risk and change if/how opioids are prescribed. When using UDT, research shows physicians often misinterpret the results and can even take drastic measures like firing a patient from their practice. The clinical advantage to the START-IT Tool is that it provides clinics with the opportunity to automatically interpret UDT within the limitations of the test, and therefore avoid misinterpretation and subsequent mismanagement.
How does it work?
The HARMS program provides clinical guidance on adapting, monitoring and prescribing strategies based on a patient’s individual level of risk. Based on results of routine UDT and other clinical observations, that risk level is then adjusted dynamically. The START-IT tool is administered on Ocean Tablets at the UDT appointments to collect and interpret all of the required information for a Point-of-care UDT (immunoassay). The result can then be utilized to dynamically adjust that patient’s risk category, and guide further monitoring and prescribing.
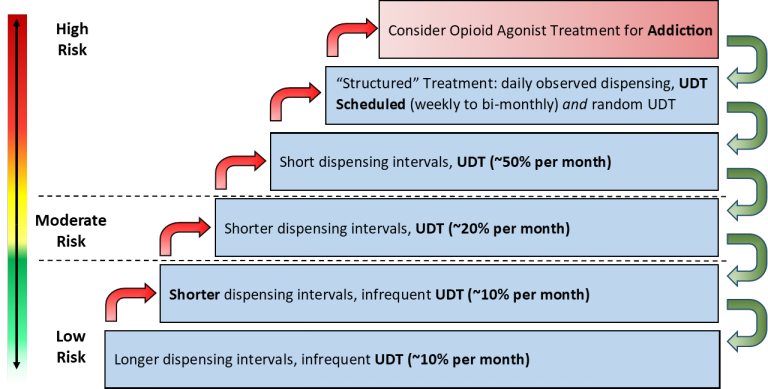
Reprinted with Permission from Safer Opioid Prescribing HARMS Program (December 2018). Summary of the HARMS Program. Marathon FHT: Dr. Ryan Patchett-Marble.
On the tablets, patients can self-report both prescribed and non-prescribed medication and the last time they took them. One of the benefits to tablets is that patients may feel more comfortable and less likely to be judged when sharing their drug history, compared to face-to-face with a nurse practitioner or clinician.
To reduce stigma even further and encourage honesty, the introduction screen reminds patients the purpose of the form is for their safety and unexpected results are less alarming if the patient acknowledges what they have been taking. Once the patient completes the form, their health care provider conducts the UDT and enters the results into the tablet. With one click, the tablet automatically interprets the results and syncs them into the EMR as an Ocean note.
The note indicates whether it is an expected or unexpected result alongside an explanation, giving the clinician a solid foundation should they need to discuss with the patient. Used in conjunction with the HARMS Program, the START-IT tool improves the practicality of UDT by automating the interpretation process and reducing the likelihood of critical misinterpretations.

Reprinted with Permission from Safer Opioid Prescribing HARMS Program (December 2018). Summary of the HARMS Program. Marathon FHT: Dr. Ryan Patchett-Marble.
The START-IT Toolkit received national recognition from the CMA’s innovation branch Joule in 2017, and recently won the 2018 CFPC Team Williams Award for IT Innovation. At AFHTO 2018, the entire team took home a Bright Lights Award for their admirable work in opioid management. Even with all of the success, Dr. Patchett-Marble remains humble about the purpose of the program. It is not intended to be punitive; patients identified as misusing opioids should not simply be removed from practice. The program is designed as a universal safety precaution to help clinicians more effectively manage and monitor the safety of their patients who rely on opioids for chronic pain – to provide support without the stigma.
To learn more about the HARMS Program, the START-IT Toolkit, or how to implement this at your clinic, visit the HARMS Program website.


